
Chlorination involves adding a measured amount of chlorine to water to produce a residual sufficient to kill bacteria, viruses, and cysts. The killing effect of chlorine depends on the pH of the water, temperature, chlorine level and contact time (i.e., the time the chlorine is in the water before consumption).
Typically, chlorine is added to public drinking water as the final stage of treatment, often following an upstream filtration step which removes sediment that can tie up chlorine and shield organisms from its effect. Chlorine has been used for over a century as a primary water disinfectant and is largely responsible for elimination of water-borne diseases such as typhoid and dysentery in developed countries.
Unfortunately, chlorine reacts with many organic compounds to form chlorine disinfection by-products that are recognized as potent carcinogens at low levels of concentrations. Such organic compounds include humic and fulvic acids, which derive from rotting vegetation common in surface waters. Reactions between free chlorine and these acids may produce a class of compounds called trihalomethanes. Strategies to reduce these in public water supplies include enhanced filtration for better organic removal and use of ammonia together with chlorine to produce chloramines for use in lieu of chlorine. Chloramines have longer half-life in the water and are less likely to produce trihalomethanes.
Chlorine is typically added to water using chemical feed systems to inject liquid sodium hypochlorite (bleach) solution or added as gaseous chlorine (typical of larger public water treatment plants).
Chlorine cannot be used in most high purity water loops without contaminating the process or interfering with the end use of the water. These systems often rely on ultraviolet disinfection as an alternative to water chlorination.
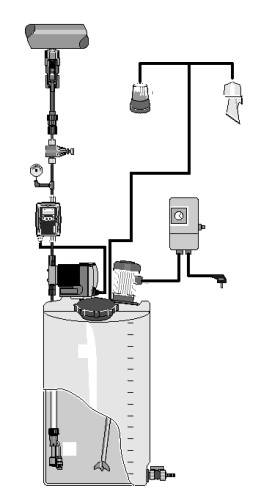
The OSEC® B-Pak system generates a 0.8 % sodium hypochlorite solution through the electrolysis of brine, consuming only water, salt and electricity. By producing hypochlorite on-site and on-demand, the system eliminates concerns associated with transportation and storage of liquefied chlorine gas or commercial sodium hypochlorite solutions, making it ideal for any application requiring chlorination.
Due to its low concentration, the hypochlorite solution generated by the OSEC B-Pak system minimizes corrosion and degradation (loss of available chlorine during storage) issues typical of high-strength (10 –15 %) sodium hypochlorite solutions. In addition, the system offers lower operating costs than commercial hypochlorite, typically resulting in attractive payback periods.
